Labels for Safety, Visuals and Facility ID Desktop Printers
Labels for Product, Wire and Lab ID Benchtop Printers
Labels for Safety, Visuals and Facility ID Desktop Printers
Labels for Product, Wire and Lab ID Benchtop Printers
Safety and Facility ID Desktop Printers
Product, Wire & Lab ID Desktop Printers
Pipe Marker Accessories & Mounting Brackets
Valve Lockouts & Hose Lockouts
Group Lock Boxes & Permit Control
PaintStripe Floor Marking Stencils
Maintenance and Production Tags
Calculators and Assessment Tools
Product Finders and Data Sheets
In environments that require complete sterility, it’s imperative that all precautions are taken to prevent external contaminants. That also goes for labeling materials, which need to meet strict sterilization standards while also standing up to the harsh chemicals and extreme temperatures of these environments. That’s why it’s important to choose high-quality sterile labels engineered for durability.
There are several unique challenges associated with labeling in sterile environments that must be addressed to ensure the integrity of products and safety of patients.

These environments require specialized labels to ensure the sterility and integrity of samples, equipment and surfaces.
Hospitals, clinics and labs require sterile environments to prevent the spread of infections and ensure patient safety; and sterilized labels play a crucial role in achieving and maintaining cleanliness.

Pharmaceutical manufacturing facilities are held to the highest standards of sterility to ensure the safety and efficacy of the drugs and vaccines they produce. In these sterile environments, specialized laboratory labels are essential for preserving product integrity and upholding stringent cleanliness standards.
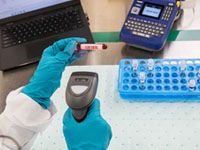
Biotechnology research centers require sterile environments for conducting experiments and handling biological samples. Specialized labels play a crucial role in these centers, serving several essential functions.

Food processing plants require sterile environments to prevent contamination and ensure food safety. Specialized labels play a crucial role in achieving these objectives.

Cleanrooms are controlled environments that maintain a high level of cleanliness and sterility. They are used in various industries, including semiconductor manufacturing, aerospace, and medical device manufacturing. Within these controlled environments, specialized labels play a vital role in ensuring the highest standards of cleanliness and sterility are consistently met.

Brady offers a range of labeling solutions specifically designed to meet the unique requirements of sterile environments — such as pharmaceutical manufacturing, medical facilities and laboratories. We understand that maintaining a sterile environment is critical to the safety of patients and the integrity of research, which is why we create labels with the highest quality of materials and a wide range of properties.

Brady’s sterile environment labels are made from specialized materials that can withstand the harsh sterilization protocols commonly found in these settings:
Our labels can be customized with labeling software to meet specific labeling requirements. Customers can choose from a wide range of sizes, shapes, colors and materials to create labels that are tailored to their unique needs.
Additionally, Brady offers a variety of printing technologies — including thermal transfer, inkjet and laser — to ensure that labels are compatible with your existing labeling equipment.
Our labels are designed with materials science in mind. Benefit from Brady’s team of scientific and engineering experts that deliver what others can’t.
Brady offers various label printing solutions to meet diverse labeling needs.

Brady's sterile environment labeling solutions offer a comprehensive range of specialized materials and customizable designs to meet the unique labeling needs of sterile environments. By utilizing Brady's labels, organizations can improve accuracy, enhance efficiency, increase safety and ensure regulatory compliance.

Streamline your lab's workflow with a Brady laboratory label printer, eliminating time-consuming handwritten labels and replacing them with fast, accurate printed labels that can import patient data.
Upgrade Your Labeling
Laboratory labels offer durable identification for the life science industry, resisting smearing, fading, and detachment even in extreme conditions like liquid nitrogen, freezers, and autoclaves. For specialized needs, design your own or collaborate with Brady for custom label solutions.
Customize Your Labels
Prevent costly errors with reliable lab labeling software that ensures accurate identification for slides, tissue cassettes, tubes, vials, and vial-top labels. Safeguard your samples, data, and personnel with precision labeling solutions.
Automate Your Lab Labeling
When considering information in the lab, almost everything involves identification. From patient data to expiration dates on chemicals, we rely on labels.
Choose the Right Lab Labels
Labeling is the cornerstone of GHS compliance. With an emphasis on consistency and comprehension of chemical labels, it is important to know what goes into a GHS compliant label for primary and secondary containers.
Understand GHS Label Compliance